Background: Allogenic hematopoietic stem cell transplantation (HCT) and ex vivo autologous gene therapies (GT) are potentially curative treatments for sickle cell disease (SCD). However, there is debate around how to define cure including what degree of donor chimerism or level of functional hemoglobin constitutes a cure. Information on red cell function post-HCT/GT and how it relates to donor chimerism and resolution of SCD complications is scarce. Red cell function tests are not currently part of follow-up care post-HCT but may provide helpful information on the degree of red cell correction and risk of SCD complications. To address this knowledge gap, we report rheology and whole blood viscosity data on HbSS patients post-HCT or GT.
Methods: Blood samples were collected under an IRB-approved protocol at Baylor College of Medicine and Emory University School of Medicine. We analyzed elongation index maximum and minimum (EImax, EImin), point of sickling (PoS), hematocrit-viscosity ratio at shear rates of 45 and 225 s-1(HVR45 & HVR225), and dense red blood cell % (DRBC%). EImax, EImin, and PoS were measured using a Laser Optical Rotational Red Cell Analyzer (Lorrca, RR Mechatronics, The Netherlands). Chimerism was measured by short tandem repeat testing (STR) after cell density sorting and DRBC% was measured on ADVIA cell counter (Siemens, Germany). HCT patients were compared with controls matched to the donor's genotype, and patients post-GT were compared with HbAS controls (HCT: HbAA donor =8, HbAS donor=19, GT=2, HbAA controls=42, and HbAS controls=15). Stata 18.0 (College Station, Texas, USA) was used to perform statistical analyses. Median values and ranges were used to describe the data. Wilcoxon rank sum test was used to compare groups and a mixed model was for the longitudinal data. A p-value <0.05 was considered statistically significant.
Results: There were 29 patients with a median age of 6.6 years (range: 2.0-16.3) at HCT/GT and a median follow-up of 2.4 years (range: 0.1-7). A total of forty-three samples were analyzed. Median donor myeloid chimerism was 94% (33-100). Overall, the HbS% ranged from 28.9 - 45.2% with HbAS donors and 0% with HbAA donors.
Two HbAS HCT patients had myeloid chimerism ≤ 50% with a follow-up period of 3.3 and 1.9 years, respectively. The myeloid donor% and HbS% were 33% and 50%, and 45.2% and 41.6%, respectively. At the first post-HCT/GT time-point (median 1 year, range: 0.1-6.9), DRBC%, EImax, and EImin were lower in HbAA donor HCT patients compared to HbAA controls (0.593 vs 0.604, p=0.008; 0.581 vs 0.604, <0.001; and 0.75 vs 0.20, p=0.02, respectively). RBC function improved with a longer follow-up from HCT/GT (median 2.9 years, range 1.3-7) with EImax showing a trend of statistical significance (p=0.07).
Patients with HbAS donors had significantly higher point of sickling (PoS) values compared to the patients with HbAA donors (p=0.02). There was a trend for a lower HVR225 in patients with HbAS donors compared to the patients with HbAA donors (p=0.08).
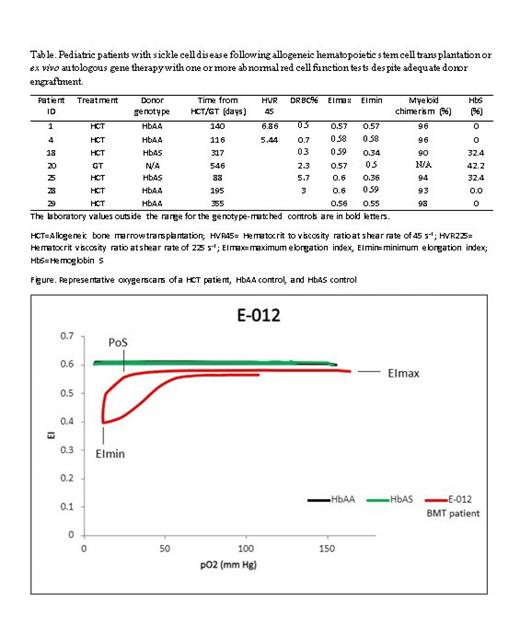
Seven patients (24%) had one or more red cell function test values outside the genotype-matched control range despite having myeloid donor chimerism of > 25-30% - a value commonly used in clinical practice to define cure (Table & Figure).
Conclusion: A substantial number of patients had abnormal red cell function tests following HCT/GT despite achieving donor engraftment. Donor myeloid chimerism ≥ 25% has been reported protective from SCD complications while a chimerism ≥50% normalized tests for hemolysis. Higher odds of SCD-related complications have been reported with higher PoS and lower EImax/EImin. Thus, we propose that red cell function should be included in follow-up care of HCT/GT patients until values normalize and clinical relevance is further assessed.
Disclosures
Stenger:bluebird bio, Inc.: Research Funding. John:bluebird bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; vertex: Membership on an entity's Board of Directors or advisory committees. Sheehan:Beam Therapeutics: Research Funding; Novartis: Research Funding; Pfizer Inc: Research Funding; Refoxy Pharmaceuticals: Research Funding; Afimmune: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal